Atrigel® Delivery System
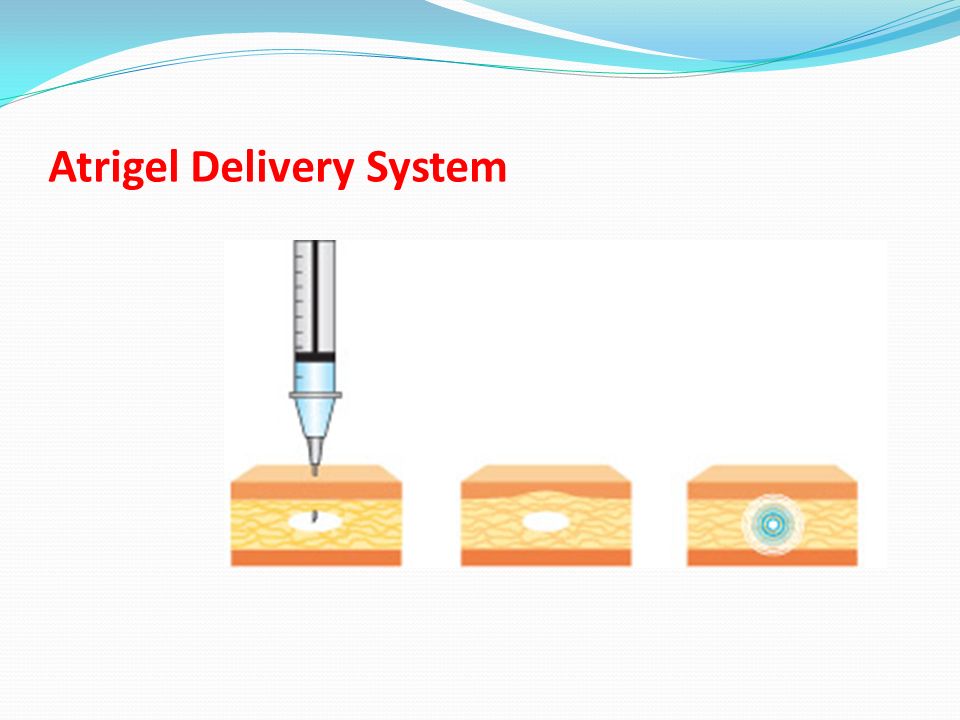
Atrigel® delivery system. All patients received a single dose of 75 mg LA suspended in the ATRIGEL Delivery System 34 ww Poly DL-lactide-co-glycolide and 66 ww N-methyl-2-pyrrolidone as a subcutaneous injection into the upper right or upper left quadrant of the abdomen. Atrigel technology designed to provide drug release in sustained manner compatibility with a broad range of pharmaceutical compounds Less invasive technique Direct de livery to a target area Protection of drug Sustained drug release. Atrigel system was initially developed by Dunn and co-workers at Southern Research Institute in Birmingham Alabama in 1987.
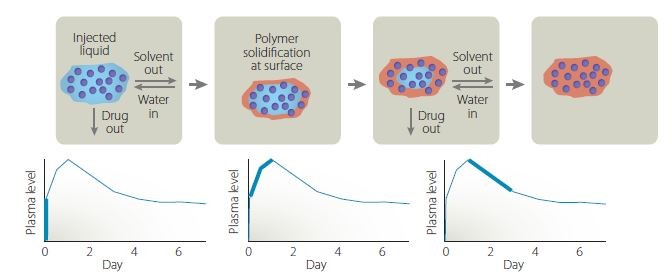
The rate of release of leuprolide acetate is controlled by varying the molecular weight of the polymer and the solvent concentrations. The technology was licensed to. Eg acute or severe bronchial asthma chronic.
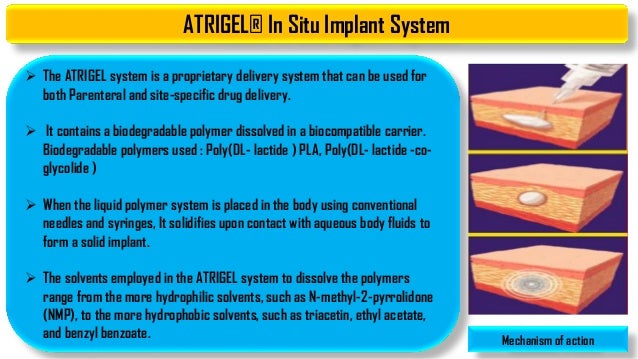
Patients with severe respiratory insufficiency. The Atrigel system is a proprietary delivery system that can be used for both parenteral and site-specific drug delivery. SUBLOCADE should not be administered to patients who are hypersensitive to buprenorphine or any component of the ATRIGEL delivery system.
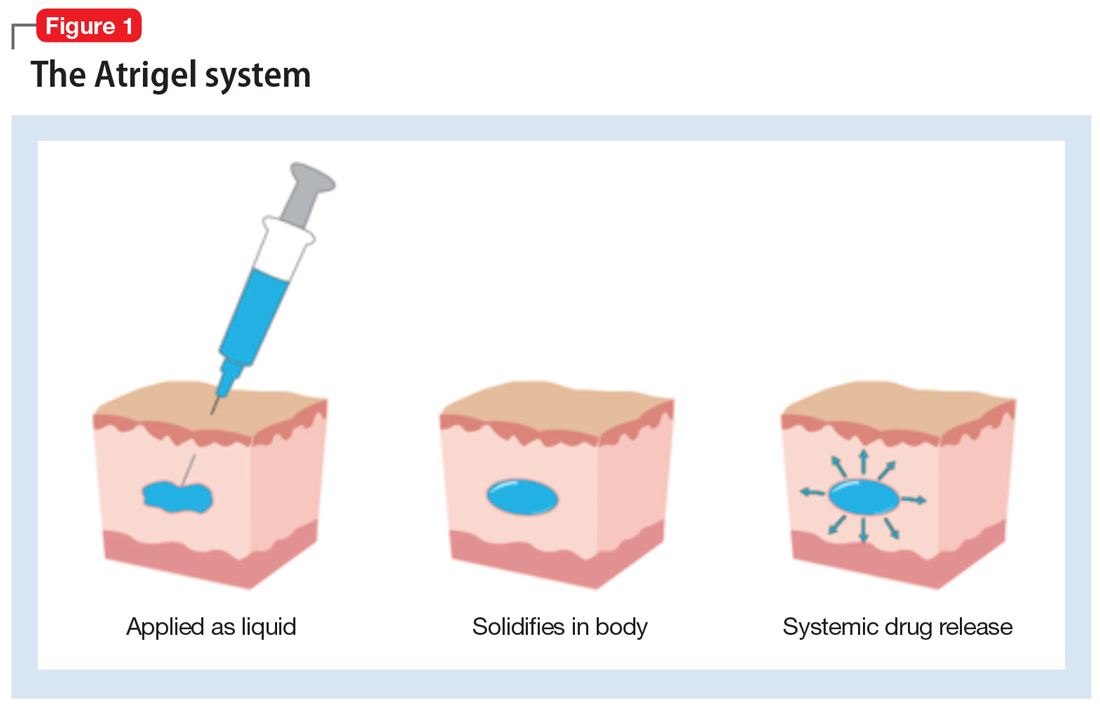
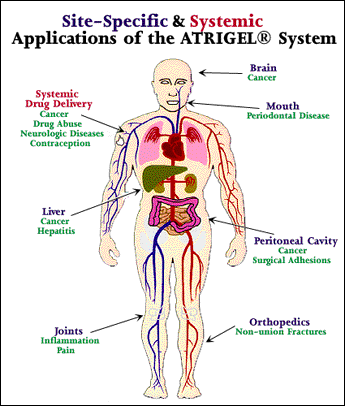
Atrigel1 The Atrigel system is a proprietary delivery system that can be used for both parenteral and site-specific drug delivery. Stay focused on patients not paperwork with TOLMARsync. Atrigel system is a proven sustained-release drug delivery platform that delivers therapeutic levels of a wide spectrum of drugs over a few days to several months with a single injection.
In the ATRIGEL Delivery System for controlled release in subgingival application ADA ACCEPTED American Dental Association Atridox has been shown to help arrest periodontitis when used as. It consists of biodegradable polymers dissolved in a biocompatible carrier. The atrigel system is a proprietary delivery system that was initially developed by Dunn and co-workers at Southern Research Institute in Birmingham Alabama in 1987.
Risperidone subcutaneous monthly injection RSQM uses Atrigel a drug delivery system utilized by several FDA-approved formulations of buprenorphine leuprolide octreotide and doxycycline for treatment of periodontitis to allow a controlled release of medication over time. Any non-medicinal ingredient or any component of the ATRIGEL Delivery System. It consists of biodegradable polymers dissolved in a biocompatible carrier.
SUBLOCADE contains buprenorphine a Schedule III controlled substance that can be abused in a manner similar to other opioids. Therefore a delivery system that combines the simplicity and reliability of solid implant devices alongwith convenience and ease of administration of microparticles is desired.
It consists of biodegradable polymers dissolved in a biocompatible carrier.
Patients with severe respiratory insufficiency. It consists of biodegradable polymers dissolved in a biocompatible carrier. The Atrigel Drug Delivery System The Atrigel system is a proprietary delivery system that can be used for both parenteral and site-specific drug delivery. In the ATRIGEL Delivery System for controlled release in subgingival application ADA ACCEPTED American Dental Association Atridox has been shown to help arrest periodontitis when used as. The rate of release of leuprolide acetate is controlled by varying the molecular weight of the polymer and the solvent concentrations. For a complete listing see DOSAGE FORMS STRENGTHS COMPOSITION AND PACKAGING 6. Doxycycline is a semi-synthetic tetracycline1 It is. ELIGARDs innovative ATRIGEL Delivery System provides controlled drug release enabling consistent suppression of testicular testosterone synthesis throughout the dosing period 1. Stay focused on patients not paperwork with TOLMARsync.
Atrigel is a novel delivery system that consists of a biodegradable polymer of DL-lactide-co-glycolic acid PLGA dissolved in the biocompatible solvent N-methyl-2-pyrrolidone. Atrigel1 The Atrigel system is a proprietary delivery system that can be used for both parenteral and site-specific drug delivery. Atrigel system is use for both parenteral and site-specific drug delivery. The Atrigel system is a proprietary delivery system that can be used for both parenteral and site-specific drug delivery. WARNINGS AND PRECAUTIONS Addiction Abuse and Misuse. The proprietary ATRIGEL Delivery System contained within ELIGARD leuprolide acetate for injectable suspension is a unique formulation technology designed to deliver a continuous dose of medicine over an extended period of time. The plans were announced last week at the companys annual general meeting as part of the firms 2007 Path to Value Creation.

































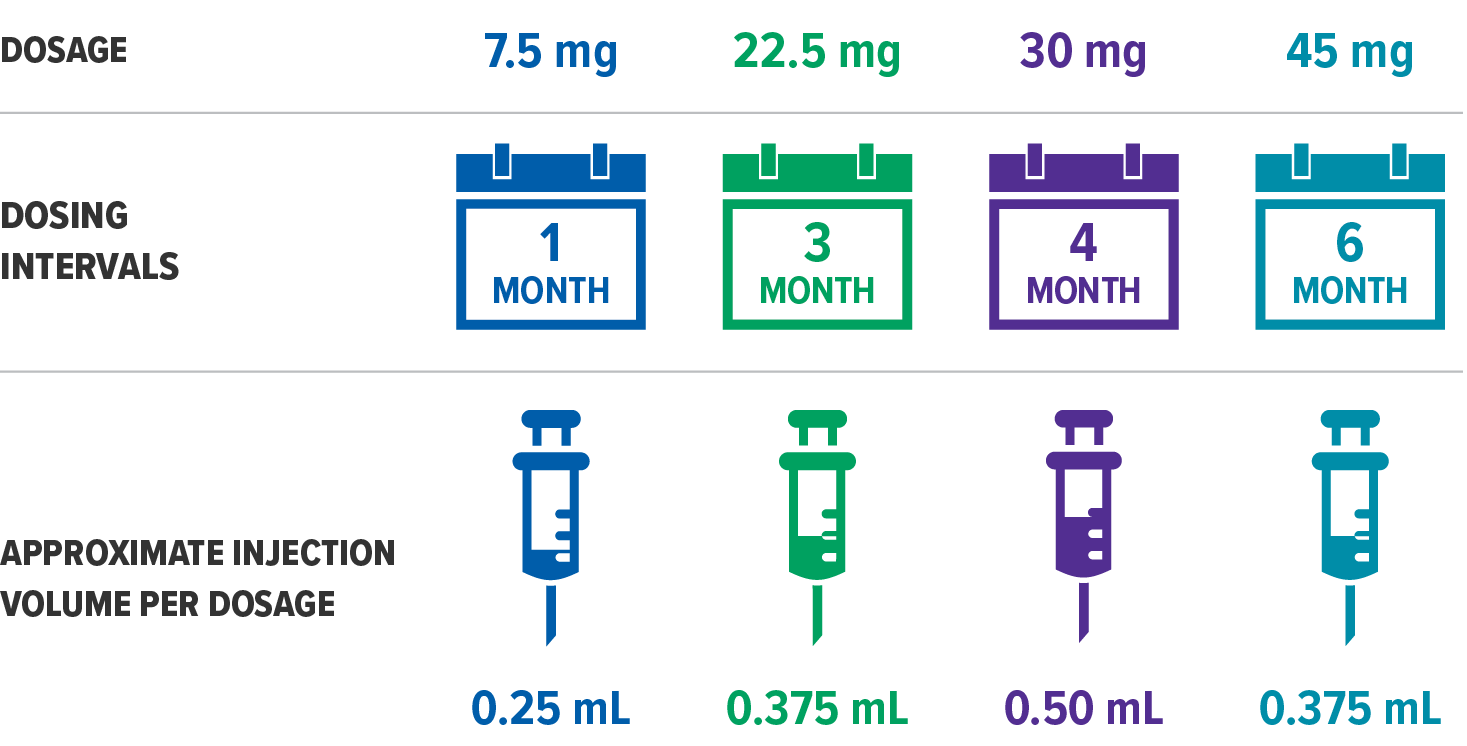
Post a Comment for "Atrigel® Delivery System"